ADVERTS
Updated Periodic Table 2018
The periodic table was created especially to organize all the elements and provide some main information about each of them. The organization is based on the increasing order of the atomic number (number of protons in the atom), the electronic configuration of the atoms of the elements, and also in order to facilitate the understanding of similar chemical properties.
If you don't know the Periodic Table very well, today you will know better what the table is, who it was created by and learn more about the elements.
ADVERTS
Updated Complete Periodic Table (click to enlarge)
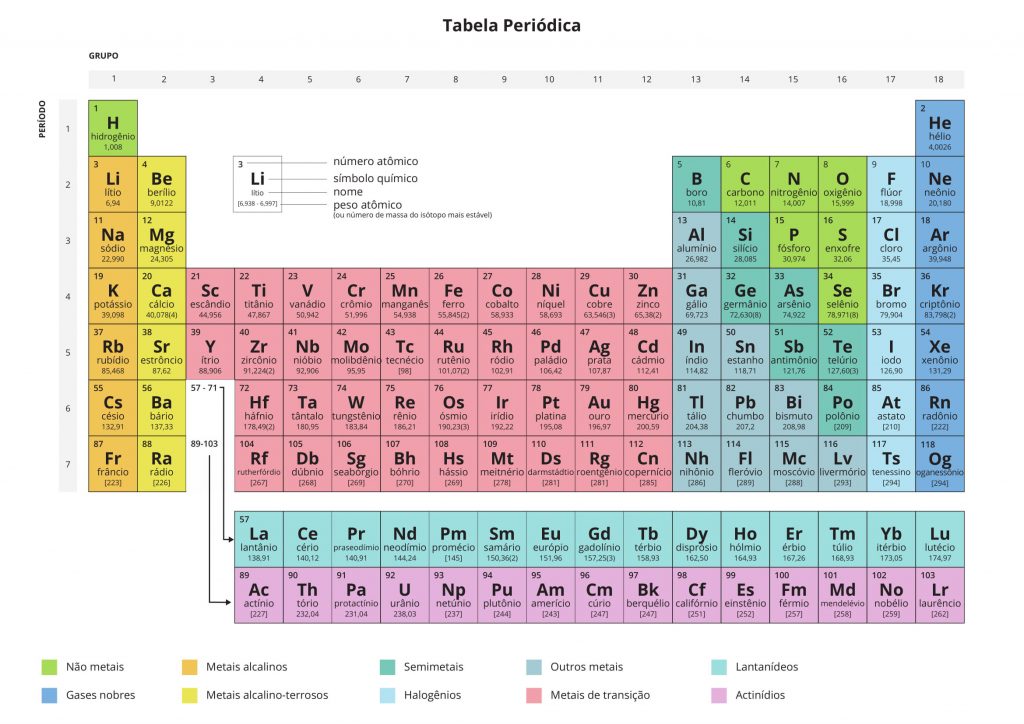
Updated Periodic Table 2018 in PDF (click to download)
Who was the creator of the Periodic Table?
The creator of the Periodic Table was Dmitri Ivanovic Mendeleev, Dmitri was a Russian physicist and chemist and even with few resources Dmitri already imagined that much more elements would exist ahead of his time, so Dmitri Mendeleev created a table that could be changed as new elements were discovered.
In what year was the Periodic Table created?
The Periodic Table was created in 1869 by physicist and chemist Dmitri Mendeleev with the aim of being able to organize all the elements discovered so far, giving the freedom to change and in this table you will also find some relevant information about each element.
How many elements are there in the Periodic Table?
ADVERTS
Over time, many elements are discovered and this causes the Periodic Table to increase over time, but to date we human beings have knowledge of 118 elements.
Classification of chemical elements in the Periodic Table
In the classification, the elements are divided into 5 groups, they are; Metals, Semimetals, Nonmetals, Noble Gases and Hydrogen.
Metals: The vast majority of chemical elements are metals, there are 87 elements in total.
Semimetals: Semimetals are a total of 7 elements, boron, silicon, germanium, arsenic, antimony and tellurium.
Nonmetals: The “a” here means “no”, that is, they can also be called non-metals, because their characteristics are totally different from those of metals, in total there are 12 elements.
Noble Gases: There are a total of 7 elements, all of these elements are found in nature and do not donate or receive electrons, as they are found in nature and do not need another element to transform, they are called noble gases.
Hydrogen: Hydrogen is also a gas, but it is not considered noble, because you can combine it with other elements, hydrogen is found in the highest layers of the atmosphere.
